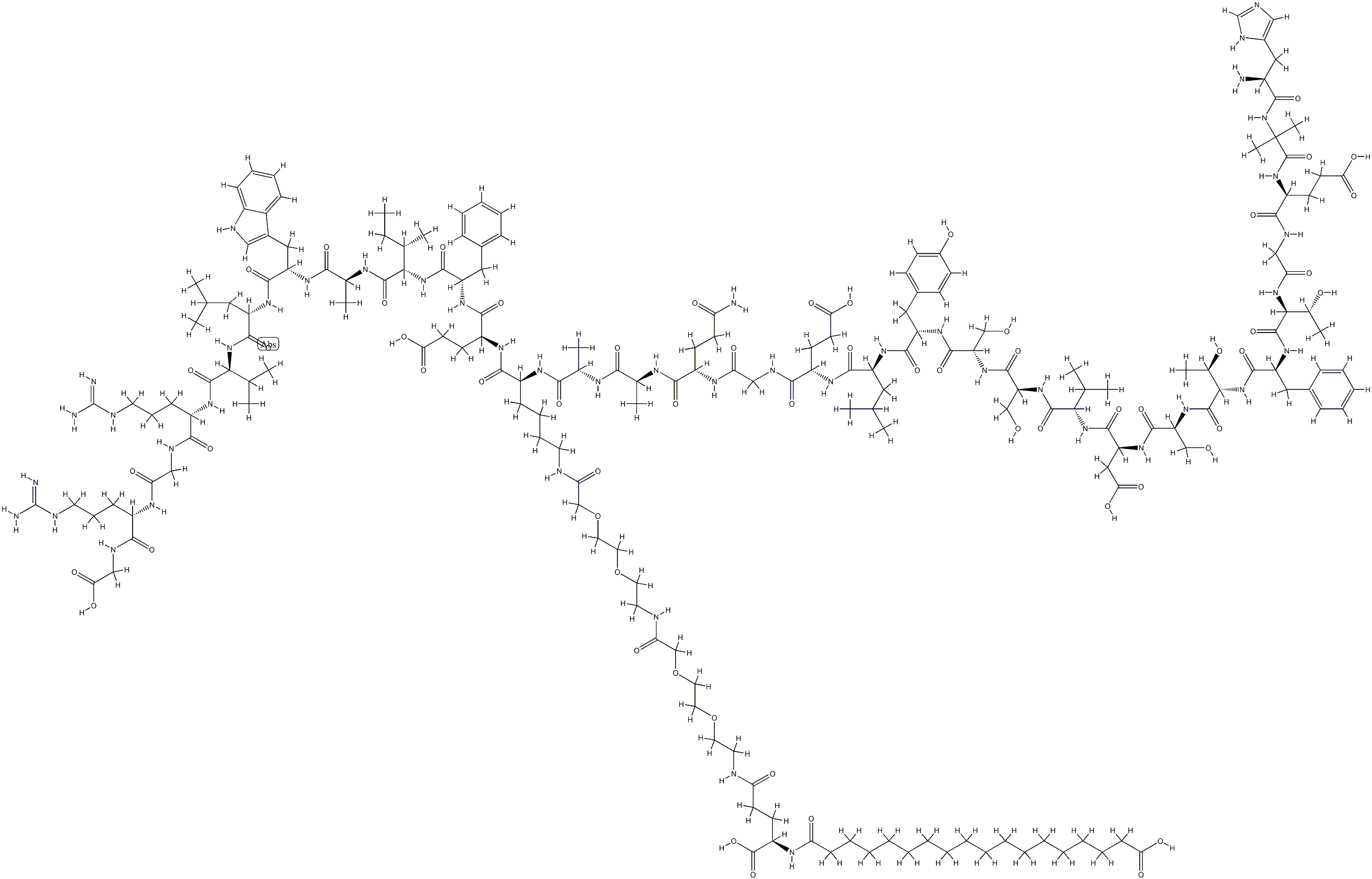The application of Semaglutide
General description
Semaglutide is a glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) with a long elimination half‐life, allowing subcutaneous (sc) administration once per week. Both the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) recently approved once‐weekly sc semaglutide for the treatment of type 2 diabetes mellitus (T2DM). The weight loss efficacy of once‐weekly sc semaglutide appears to be superior compared with the other once‐weekly GLP‐1 RAs in patients with T2DM. Semaglutide was recently evaluated as an antiobesity drug in a phase II dose‐finding trial, which demonstrated superior weight loss efficacy of once daily ssemaglutide compared with both placebo and once daily 3.0 mg liraglutide in patients with obesity but without T2DM. The magnitude of semaglutide‐induced weight loss in this study exceeded the criteria of both the EMA and FDA for antiobesity drugs, and there were no safety concerns, indicating the eligibility of once dailysc. semaglutide as a future antiobesity drug.
Application and Pharmacology
1.Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes: A total of 3183 patients were randomly assigned to receive oral semaglutide or placebo. The mean age of the patients was 66 years; 2695 patients (84.7%) were 50 years of age or older and had cardiovascular or chronic kidney disease. The median time in the trial was 15.9 months. Major adverse cardiovascular events occurred in 61 of 1591 patients (3.8%) in the oral semaglutide group and 76 of 1592 (4.8%) in the placebo group (hazard ratio, 0.79; 95% confidence interval [CI], 0.57 to 1.11; P<0.001 for noninferiority). Results for components of the primary outcome were as follows: death from cardiovascular causes, 15 of 1591 patients (0.9%) in the oral semaglutide group and 30 of 1592 (1.9%) in the placebo group (hazard ratio, 0.49; 95% CI, 0.27 to 0.92); nonfatal myocardial infarction, 37 of 1591 patients (2.3%) and 31 of 1592 (1.9%), respectively (hazard ratio, 1.18; 95% CI, 0.73 to 1.90); and nonfatal stroke, 12 of 1591 patients (0.8%) and 16 of 1592 (1.0%), respectively (hazard ratio, 0.74; 95% CI, 0.35 to 1.57). Death from any cause occurred in 23 of 1591 patients (1.4%) in the oral semaglutide group and 45 of 1592 (2.8%) in the placebo group (hazard ratio, 0.51; 95% CI, 0.31 to 0.84). Gastrointestinal adverse events leading to discontinu ation of oral semaglutide or placebo were more common with oral semaglutide. In this trial involving patients with type 2 diabetes, the cardiovascular risk profile of oral semaglutide was not inferior to that of placebo[1].
2. Semaglutide as a promising antiobesity drug: Current evidence clearly supports the superior weight loss efficacy of once‐weekly sc semaglutide compared with the other once‐weekly GLP‐1 RAs with a similar safety profile. Once daily sc semaglutide has recently emerged as a promising treatment option for obesity with possibly greater efficacy than the existing antiobesity drugs and with acceptable safety profile. The addition of semaglutide to the armamentarium for the treatment of obesity is reasonably expected to provide more and better antiobesity therapeutic options and substantially contribute to the establishment of a high level of precision medicine in the management of obesity.104 The exact role of semaglutide in the treatment of obesity remains to be established in future studies with greater numbers of participants and extended duration of follow‐up[2].
Figure 1 Crystal structure of the semaglutide peptide backbone (gray) in complex with the GLP-1 receptor extracellular domain (golden surface). Individual residues are shown as sticks, with nitrogen and oxygen atoms colored blue and red, respectively[3].
Safety
The glucagon-like peptide-1 receptor agonist (GLP-1RA) semaglutide is the most recently approved agent of this drug class, and the only GLP-1RA currently available as both subcutaneous and oral formulation. While GLP-1RAs effectively improve glycemic control and cause weight loss, potential safety concerns have arisen over the years. For semaglutide, such concerns have been addressed in the extensive phase 3 registration trials including cardiovascular outcome trials for both subcutaneous (SUSTAIN: Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) and oral (PIONEER: Peptide InnOvatioN for the Early diabEtes tReatment) semaglutide and are being studied in further trials and registries, including real world data studies. In the current review we discuss the occurrence of adverse events associated with semaglutide focusing on hypoglycemia, gastrointestinal side effects, pancreatic safety (pancreatitis and pancreatic cancer), thyroid cancer, gallbladder events, cardiovascular aspects, acute kidney injury, diabetic retinopathy (DRP) complications and injection-site and allergic reactions and where available, we highlight potential underlying mechanisms. Furthermore, we discuss whether effects are specific for semaglutide or a class effect. It conclude that semaglutide induces mostly mild-to-moderate and transient gastrointestinal disturbances and increases the risk of biliary disease (cholelithiasis). No unexpected safety issues have arisen to date, and the established safety profile for semaglutide is similar to that of other GLP-1RAs where definitive conclusions for pancreatic and thyroid cancer cannot be drawn at this point due to low incidence of these conditions. Due to its potent glucose-lowering effect, patients at risk for deterioration of existing DRP should be carefully monitored if treated with semaglutide, particularly if also treated with insulin. Given the beneficial metabolic and cardiovascular actions of semaglutide, and the low risk for severe adverse events, semaglutide has an overall favorable risk/benefit profile for patient with type 2 diabetes[4].
Reference
1.Husain M., Birkenfeld A. L. & Donsmark M. et al., "Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes," The New England journal of medicine, Vol.381, No.9(2019), pp.841-851.
2.Christou G. A., Katsiki N. & Blundell J. et al., "Semaglutide as a promising antiobesity drug," Obesity Reviews, Vol.20, No.6(2019), pp.805-815.
3.Knudsen L. B. & Lau J., "The Discovery and Development of Liraglutide and Semaglutide," Frontiers in Endocrinology, Vol.10(2019).
4.Smits M. M. & Van Raalte D. H., "Safety of Semaglutide," Frontiers in Endocrinology, Vol.12(2021).
You may like
Related articles And Qustion
See also
Lastest Price from Semaglutide manufacturers

US $5.00/mg2026-02-02
- CAS:
- 910463-68-2
- Min. Order:
- 10mg
- Purity:
- 99% HIGH PURITY
- Supply Ability:
- 5000tons

US $10.00/BOX2026-01-31
- CAS:
- 910463-68-2
- Min. Order:
- 1BOX
- Purity:
- 99%
- Supply Ability:
- 10000